In recent years, the detection rate of pulmonary nodule lesions has increased significantly. Clinically, lesions within the lung parenchyma that are less than or equal to 3 cm (excluding atelectasis and enlarged lymph nodes) are called nodules, lesions larger than 3 cm are called masses, and nodules smaller than 1 cm are called small nodules, and those smaller than 5 mm are called micronodules. The reason for this definition is that lesions larger than 3 cm are mostly malignant, while smaller lesions may be benign or malignant. There is a correlation between the size of the nodule and the benign or malignant nature of the nodule. Small nodules are more likely to be benign.
There are several types of people who should not take it lightly when they find small lung nodules during physical examinations. They should actively receive formal further examinations and timely treatment:
(1) People who have smoked for more than 20 years, smoke more than 20 cigarettes a day, or have been exposed to passive smoking for a long time;
(2) People who are over 40 years old and have symptoms such as chest pain, cough, unexplained blood in sputum, emaciation, weight loss, etc.;
(3) People with a family history of tumors, especially a family history of lung cancer;
(4) Nodules larger than 1 cm in size, accompanied by changes such as spicule-like, lobed or ground-glass-like, and pleural indentation.
In chest CT examination, nodules smaller than 5 mm are too small to have obvious characteristics of benign or malignant, and various examinations are difficult to determine their nature. More than half of single small nodules in the lungs with a diameter greater than 1 cm are malignant, while more than 90% of tiny nodules smaller than 5 mm are benign. Therefore, lung nodules smaller than 5 mm can be reexamined by CT every 6 months; lung nodules of 5-10 mm should be reexamined by CT every 3 months; and lung nodules of 10 mm should be reexamined by CT every 1 to 2 months. If the follow-up finds no change in the lesion, the reexamination is often extended to 3-6 months or 1 year. If a small lung nodule is found to have no change for 2 years, it can be generally considered a benign nodule. When changes are found in the lesion, the next step of treatment is determined based on the changes.
The Fleischner Society Guidelines (2017) recommend the selection of 6 mm (100 mm3) as the main cutoff value for shortening the follow-up interval. For multiple solid nodules, if the size is<6 mm (100 mm3), if classified as high risk, follow-up is recommended at 12 months, otherwise no routine follow-up is required; for those with the main lesion ≥6 mm (100 mm3), follow-up is recommended for 3 to 6 months, and a second follow-up is selectively performed at 18 to 24 months. For multiple subsolid nodules, follow-up for 3 to 6 months is recommended, and subsequent treatment is determined based on the most suspicious nodule. Among them, multiple pGGNs <6 mm are mostly benign, but high-risk patients are recommended to be followed up in the second and fourth years.
Early testing, early intervention, and early treatment effectively improve our understanding of lung conditions.
With the vision of "providing a third way of dosing", Feellife® focuses on the field of medical nebulization and respiratory health. With its strong scientific research capabilities and deep insight into user demands, it has devoted itself to the research and development of more than 220 atomization patents with AiMesh® as the core for more than ten years, and has pioneered the development of intelligent variable frequency atomization technology, quantitative atomization technology, and respiratory sensing atomization technology, all of which have been successfully applied to atomizers. Through the business model of "intelligent nebulizer + nebulization liquid", it provides atomization solutions to the world and serves the global atomization ecosystem.
At the same time, feellife's pulmonary function testing equipment obtained FDA certification in 2023, adding luster to the creation of a closed loop of respiratory and lung health prevention, treatment and rehabilitation.
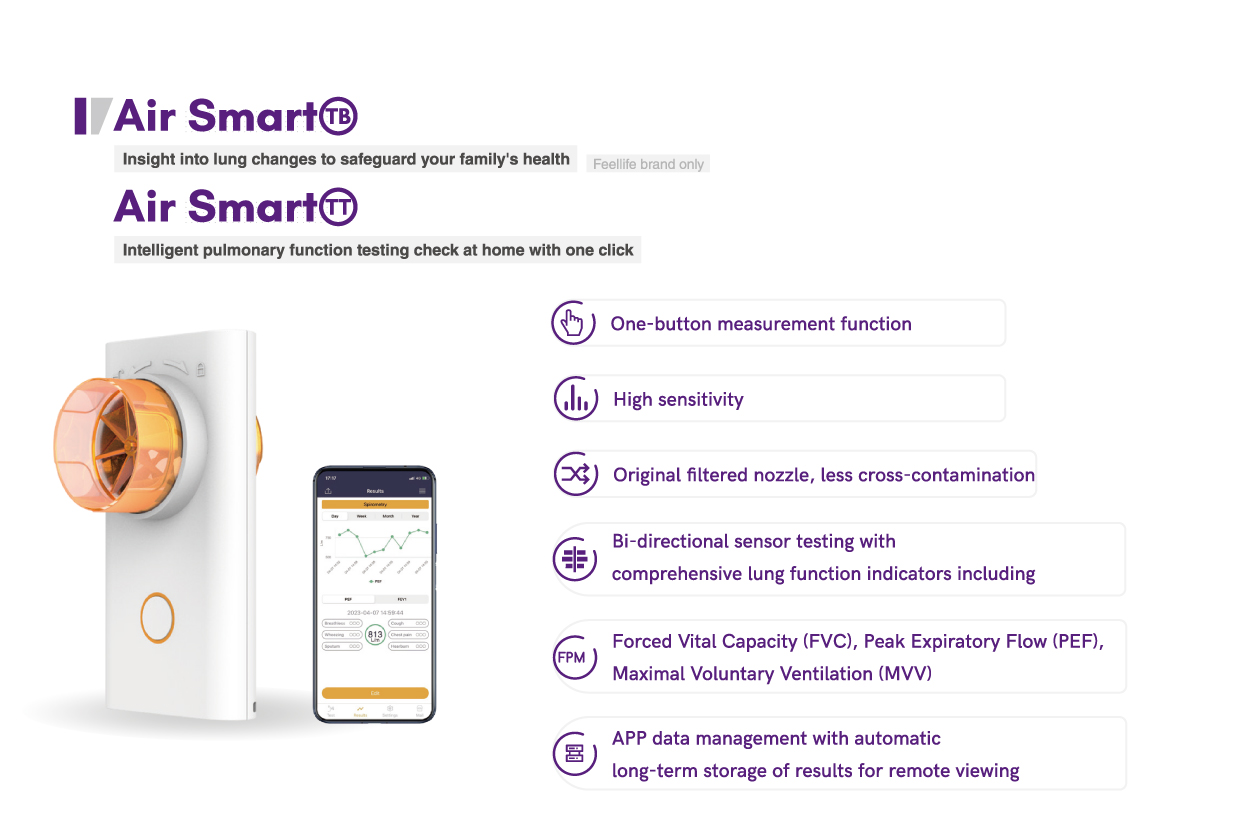
We have launched a "three-piece set of tuberculosis rehabilitation" treatment plan, including the Air Smart TT lung function test, the fit series of lung training, and the Airbar Pro 1 oxygen concentrator. For home use, you can choose a more compact and portable portable respiratory measurement device. With one blow every day, you can monitor your health in real time. The mobile phone APP generates health data, and trend changes are clear at a glance.
In addition, you can perform lung function training every day. Try the portable Airfit1 at home to train your respiratory muscles, increase your respiratory and cardiopulmonary functions, make it easier to breathe during exercise, and make lung exercise easier.

As a home oxygen concentrator, the main function of Airbar Pro 1 is to provide users with pure medical oxygen, improve symptoms such as dyspnea and hypoxia, and improve the quality of life. At the same time, the oxygen content can be as high as 93%.
