Management of acute exacerbations in emergency care primarily depends on nebulizers. Although jet nebulizers (JN) have been traditionally used, vibrating mesh nebulizers (VMN) are now being implemented.
A study compares aerosols generated by VMN with NIV and pulmonary deposition and distribution across regions of interest with administration of radiolabeled aerosol with jet nebulizer (JN) during NIV.

Methods
A crossover single dose study involving 9 stable subjects with moderate to severe COPD randomly allocated to receive aerosol administration by the VMN and the jet nebulizer operating with oxygen at 8 lpm during NIV. Radiolabeled bronchodilators (fill volume of 3 mL: 0.5 mL salbutamol 2.5 mg + 0.125 mL ipratropium 0.25 mg and physiologic saline up to 3 mL) were delivered until sputtering during NIV (pressures of 12 cmH2O and 5 cmH2O - inspiratory and expiratory, respectively) using an oro-nasal facemask. Radioactivity counts were performed using a gamma camera and regions of interest (ROIs) were delimited. Aerosol mass balance based on counts from the lungs, upper airways, stomach, nebulizer, circuit, inspiratory and expiratory filters, and mask were determined and expressed as a percentage of the total.
Results
Both inhaled and lung doses were greater with VMN (22.78 ± 3.38% and 12.05 ± 2.96%, respectively) than JN (12.51 ± 6.31% and 3.14 ± 1.71%; p = 0.008). Residual drug volume was lower in VMN than in JN (3.08 ± 1.3% versus 46.44 ± 5.83%, p = 0.001). Peripheral deposition of radioaerosol was significantly lower with JN than VMN.
Intragroup analysis of radioaerosol pulmonary index for ROI across vertical and horizontal gradients is shown in Table below
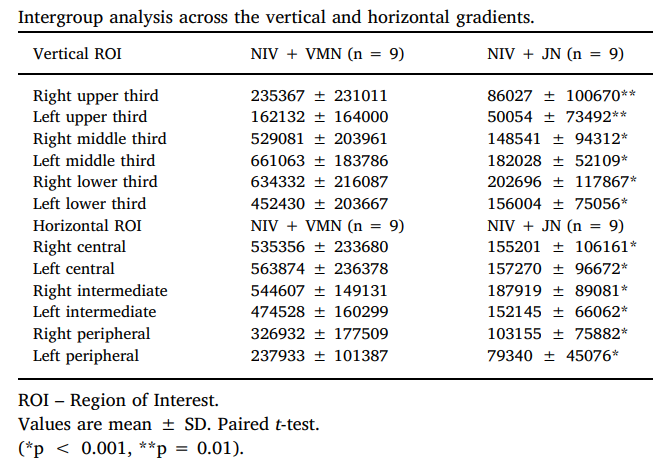
When analyzing intergroup deposition in different ROI across vertical and horizontal gradients, we found that NIV + VMN group had higher counts in comparison to NV + VMN group, as shown in Table below.
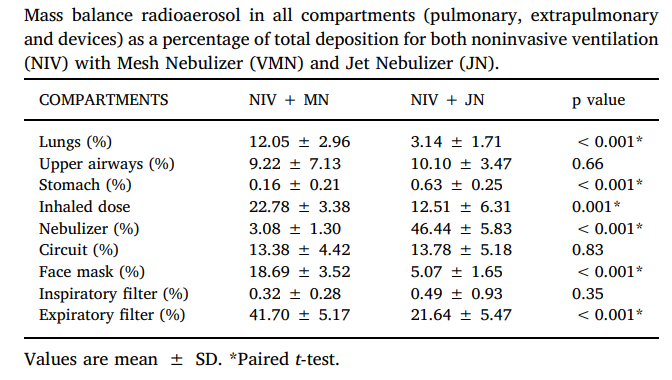
The percentage of inhaled mass was significantly higher in NIV + VMN group when compared to NIV + JN group (19.90 ± 3.18% versus 7.03 ± 2.97%, p = 0.008). VMN presented a lower residual volume (3.20 ± 1.33% versus 48.53 ± 6.40%, p = 0.008) and more radioaerosol deposited in the face mask and upper airways, in comparison to jet nebulizer during NIV. The JN demonstrated greater deposition in the expiratory filter than the VMN device. No differences were found regarding to mass balance found in the stomach, circuit and inspiratory filter for either group. Table represent the mass balance obtained in each compartment from both groups and mass balance of aerosol across pulmonary, extrathoracic and device compartments.
Conclusion
This study found more than 3-fold aerosolized particles from the total dose of 3 mL of solution charged into the VMN in comparison to JN. Further randomized controlled trials are necessary to evaluate clinical response to the increased level of aerosol deposition obtained from VMN and its impact to relieve respiratory discomfort and dynamic hyperinflation in COPD, as well as to develop recommendations for the use of this resource.
Reference
[1] Global Initiative for Chronic Obstructive Lung disease (GOLD), Global Strategy for Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease, (2013) available from: http://www.goldcopd.org/ , Accessed date: 3 April 2013.
[2] P. Haidl, S. Heindl, K. Siemon, M. Bernacka, R.M. Cloes, Inhalation device requirements for patients' inhalation maneuvers, Respir. Med. 118 (2016) 65–75.
[3] D. Hochrainer, H. Hӧlz, C. Kreher, L. Scaffidi, M. Spallek, H. Wachtel, Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers, J. Aerosol Med. 18 (3) (2005 Fall) 173–282.
[4] V.G. Press, V.M. Arora, L.M. Shah, S.I. Lewis, K. Ivy, J. Charbeneau, et al., Misuse of respiratory inhalers in hospitalized patients with asthma or COPD, J. Gen. Intern.Med. 26 (6) (2011 Jun) 635–642.
[5] W. Vincken, P.R. Dekhuijzen, P. Barners, The ADMIT series – Issues in inhalation therapy. 4) How to choose inhalers devices for the treatment of COPD, Prim. Care Respir. J. 19 (1) (2010 Mar) 10–20.
[6] C. Leach, G.L. Colice, A. Luskin, Particle size of inhaler corticosteroids: Does it matter? Allergy Clin. Immunol. 124 (6 Suppl) (2009 Dec) S88–S93.